Dr. Isacoff recently presented Chemotherapy for Pancreatic Cancer Patients: Less is More! where he shared new information about how the dosing and frequency of chemotherapy may affect patient outcomes. Watch below:
About Pancreatic Cancer

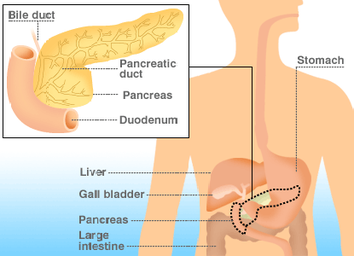
Pancreatic cancer develops when abnormal cells in the pancreas grow out of control, forming a tumor, or a mass. The overwhelming majority of pancreatic cancers – 95% - are exocrine tumors. Exocrine cells in the pancreas produce enzymes that aid in digestion.
Neuroendocrine tumors (also known as endocrine or islet cell tumors) account for less than 5% of pancreatic cancers. Islet cells are endocrine cells in the pancreas that produce and secrete insulin, glucagon and somatostatin into the blood stream to regulate blood sugar levels and other hormones in the blood. Neuroendocrine tumors may be benign or malignant and tend to grow slower than exocrine tumors.
Pancreatic cancer is the fourth deadliest type of cancer. Despite new drug treatments, survival rates for pancreatic cancer remain the same as they were 40 years ago. According to the Pancreatic Cancer Action Network, 94% of patients die within five years of diagnosis.
Dr. Isacoff is helping patients beat these odds with treatments that eliminate radiation and combine chemotherapy drugs delivered at low-doses for more effective treatment.
Neuroendocrine tumors (also known as endocrine or islet cell tumors) account for less than 5% of pancreatic cancers. Islet cells are endocrine cells in the pancreas that produce and secrete insulin, glucagon and somatostatin into the blood stream to regulate blood sugar levels and other hormones in the blood. Neuroendocrine tumors may be benign or malignant and tend to grow slower than exocrine tumors.
Pancreatic cancer is the fourth deadliest type of cancer. Despite new drug treatments, survival rates for pancreatic cancer remain the same as they were 40 years ago. According to the Pancreatic Cancer Action Network, 94% of patients die within five years of diagnosis.
Dr. Isacoff is helping patients beat these odds with treatments that eliminate radiation and combine chemotherapy drugs delivered at low-doses for more effective treatment.